Instructions for IRB Members
- Log in to ARROW (http://arrow.wisc.edu) using your NetID and password.
- Once logged in, make sure the “My Roles” bar in the top left displays “IRB Member” as your role. If it does not, select “IRB Member” from the drop-down menu under “My Roles.”
- You are now in your IRB Member homepage.
Print Meeting Agenda
- Click on the “Meetings” tab in your IRB member homepage. You may need to click on the “…” tab to open a drop-down menu before you can select “Meetings.”
- Click on the meeting for which you wish to review and print the agenda. You are now in the meeting workspace.
- Click on the button labeled “Print Open Agenda” to print a hard copy of the open agenda.
- Click on the button labeled “Print Closed Agenda” to print a hard copy of the closed agenda.
- NOTE You can access any agenda items in the closed agenda by clicking on the ID number. If you are not assigned as primary reviewer, this is how you can access submissions to review them for an upcoming meeting.
- To return to your IRB member homepage, click on “My Home” in the upper left corner.
Reviewing Initial Application submissions
- In your IRB member workspace, check your “HS/MR: Initials” or “ED/SBS: Full” tab for submissions assigned to you as one of the primary reviewers.
- Click on the title of the submission in your Inbox to access the submission workspace and begin your review.
- Otherwise, access the submissions being reviewed in a meeting from the agenda.
- Once in the submission workspace, the upper-left should indicate that the submission is in the “Assigned to IRB Meeting” state. The submission title should be displayed near the top of the screen.
- Click on the button labeled “View Application” to review the initial application SmartForm. Use the SmartForm features, including the “Continue” button and the “Jump To” menu to navigate the form.
NOTE Use the “Jump To” menu to go back to previous pages of the SmartForm rather than using the back button on your internet browser. - Once you have finished reviewing the protocol, application, and supplemental documents uploaded by the research team (e.g. consent forms, study protocol, questionnaires, recruitment materials), you can exit the application using the “Exit” button near the top of the screen. This will take you back to the submission workspace.
- Any required checklists will be completed during the IRB meeting.
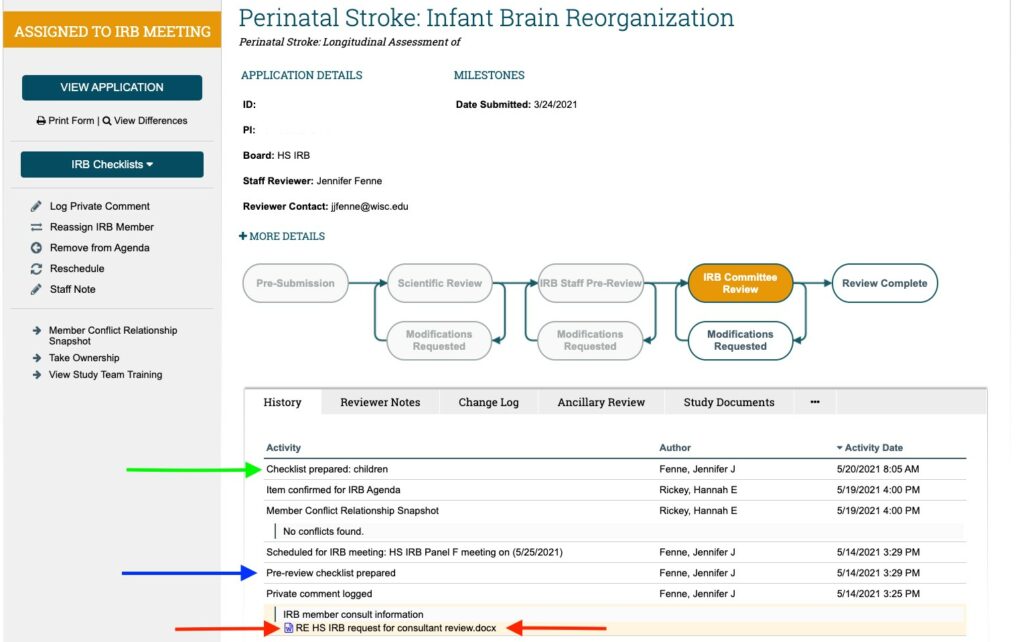
- In the History tab, select “Pre-review checklist prepared” to view HRP-401, the pre-review checklist completed by the staff reviewer. In the screenshot below, this is pointed out with a blue arrow.
- Within the checklist, pay attention to Section #5, “Notes.” This section includes a short summary of the study, important regulatory details, and any problematic issues.
- Also view any other toolkit checklists that have been completed by the staff reviewer. You will see them in the History tab as an activity beginning with, “Checklist prepared:” In the screenshot below, this is pointed out with a green arrow.
- Review any other relevant information in the History tab. As an example, in the screenshot below, red arrows point to a Word document uploaded as part of a private comment labeled, “IRB member consult information.” This contains feedback from an IRB Member with relevant specialty expertise who won’t be present at the meeting.
- For now, as you review these checklists, do NOT execute any activities yourself. Any required checklists will be completed during or after the IRB meeting by the staff reviewer. We will notify you if and when this expectation changes.
- To remind yourself of the required regulatory criteria for approval, use HRP-314 WORKSHEET: Criteria for Approval for a guide.
- Find this by selecting “Worksheets” under the “Toolkit Library” tab on this website.
- On the page of Toolkit worksheets, select #314, Criteria for Approval.
- Within HRP-314, review Section 2 to assess the “111 criteria” (criteria for approving a human subjects research study). You do NOT need to complete this worksheet but it is there as a guide.

- During the IRB meeting, when discussing the study it is NOT necessary to go through each of these criteria. You can focus on areas that require discussion, and can state that, “other 111 criteria have been met.”
- Also within HRP-314, use Section 5 and 7 to review the regulatory requirements for informed consent.
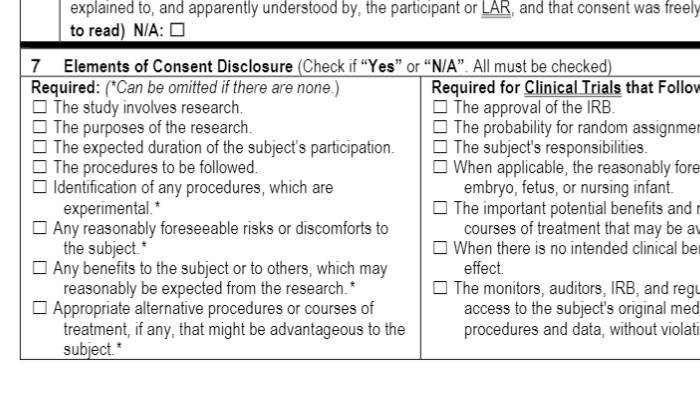
- During the IRB meeting, it is NOT necessary to go through each of these criteria. Simply note any waivers or alterations of informed consent that need to be granted, as well as deficiencies in the consent materials.
- If you have questions about the content of the study, contact the assigned staff reviewer, the PI of the study if you are comfortable, your panel administrator, or one of the IRB Member Points of Contact.
Who to Contact
- Help with navigating and using ARROW
- Contact the IRB Member Point of Contact, Jackie Lee at (608) 261-1157 or Jessie Johnson at (608) 263-0835
- Questions about a specific protocol
- Contact the assigned staff reviewer