Overview of GCP updates
On October 15, 2025, the CITI Program updated the modules in UW–Madison’s “Good Clinical Practice for Drug/Device Researchers” basic and refresher courses to reflect the ICH E6(R3) guidelines, which were adopted by the FDA.
UW-Madison does not require that study personnel update their training until their existing training expires. Details about UW-Madison’s GCP training requirements can be found on the Good Clinical Practice (GCP) Training Guidance and Instructions page.
Individual research sponsors may require that study personnel update their GCP training prior to their existing training expiration. In order to get an updated course completion date (and extend the training expiration date an additional three years), study personnel should review and follow the below information.
To check whether the training you completed refers to ICH E6(R2) or ICH E6(R3), see CITI’s help page. Our “Good Clinical Practice for Drug/Device Researchers” courses align with the “GCP for Clinical Trials with Investigational Drugs and Medical Devices (U.S. FDA Focus)” and “GCP FDA Refresher”.
For questions about GCP training, contact citisupport@research.wisc.edu.
How to complete the updated GCP training
Learners should complete the following instructions in order to receive an updated course completion date (and extended expiration date).
- Determine if you most recently completed the “Basic Course” or a “Refresher Stage”
- Log into the UW CITI Portal (https://irb.research.wisc.edu/CITI).
- Click “My Records” at the top of the page (see Screenshot 1 at the bottom of this page).
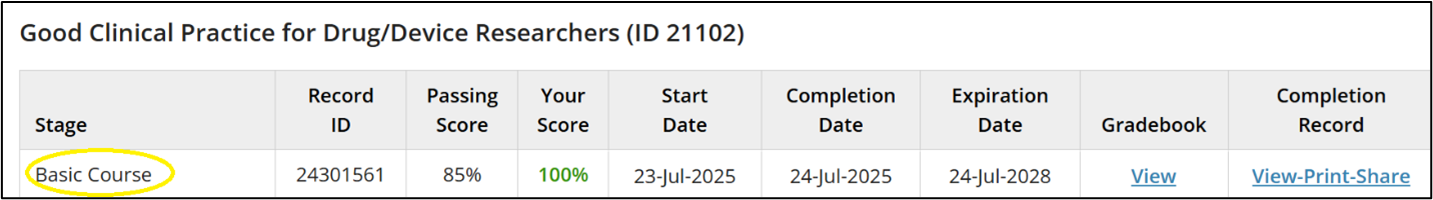
- Scroll through your records until you find “Good Clinical Practice for Drug/Device Researchers” and determine whether you most recently completed the “Basic Course” or a “Refresher Stage” (see Screenshot 2 at the bottom of this page).
- Based on what you determined in Step 1, follow the applicable instructions set
- If you most recently completed the “Basic Course”, complete this brief form to request that the refresher course be added to your account. Requests may take several days to fully process. You will receive additional instructions once the request is processed.
- If you most recently completed the “Refresher Stage”, you will complete the following steps to add the Basic Course to your account. Completing the newly added Basic Course will result in an updated completion certificate.
- Click “My Courses” at the top of the page (see Screenshot 3 at the bottom of this page), then click “View Courses” next to University of Wisconsin-Madison.
- Scroll to the bottom and click on “Remove a Course” under “Learner Tools for University of Wisconsin – Madison” (see Screenshot 4 at the bottom of this page).
- Check the box next to “Good Clinical Practice for Drug/Device Researchers” and press “Submit”. If needed, click “View Courses” next to University of Wisconsin – Madison again.
- Now click “Add a Course” under “Learner Tools for University of Wisconsin – Madison” to re-add the course by checking the box next to “Good Clinical Practice for Drug/Device Researchers” and pressing “Submit”. The “Good Clinical Practice for Drug/Device Researchers” course should now state “Stage 1 – Basic Course” and be ready to begin. If it does not, please contact citisupport@research.wisc.edu.
- Click “My Courses” at the top of the page (see Screenshot 3 at the bottom of this page), then click “View Courses” next to University of Wisconsin-Madison.
Screenshot 1: My Records

Screenshot 2: Determining which stage you most recently completed
Screenshot 3: My Courses

Screenshot 4: Learner Tools for “Add a Course” and “Remove a Course”
